
Luísa C. Roseiro and Carlos Santos
Instituto Nacional de Investigação Agrária e Veterinária, I.P., Oeiras, Portugal
INTRODUCTION
Carnitine (β-hydroxy-γ-trimethylaminobutyrate), a nonessential organic nutrient, is a quaternary ammonium compound, naturally occurring in nature. It is found in greater concentration in all animal species, and in numerous microorganisms and plants (Vaz and Wanders, 2002). l-carnitine has a number of important roles in intermediary metabolism. l-carnitine is involved in the transport of activated long-chain fatty acids from the cytosol to the mitochondrial matrix, where β-oxidation takes place. Other physiological roles of carnitine include modulation of the acyl-CoA/CoA ratio (Carter et al., 1995; McGarry and Brown, 1997), storage of energy as acetyl-carnitine (Bremer, 1983; Carter et al., 1995), and the modulation of toxic effects of poorly metabolized acyl groups by excreting them as carnitine esters (Duran et al., 1990; Rebouche, 1996). Aside from assisting in fatty acid transport, carnitine has an antioxidant activity, protecting various cells against oxidative injury (Ribas et al., 2014). In animal tissues, l-carnitine is maintained by absorption from dietary sources, endogenous synthesis, and efficient tubular reabsorption by the kidney. The main sources of dietary l-carnitine include animal products, particularly red meat with 500–1200 mg/kg, followed by fish, chicken, and dairy products, containing 16–64 mg/kg. On the other hand vegetables, fruits, and grains contain very little carnitine amount (<0.5 mg/kg). Unfortunately, only 60%–70% of available carnitine is absorbed from food sources and its content can be depleted if meat is cooked at high temperature over an open flame (Bloomer et al., 2013). Although animals obtain carnitine primarily from their diet, most mammals are capable of synthesizing carnitine endogenously. Synthetized from essential amino acids, lysine and methionine, l-carnitine is involved in reversible transesterification reactions with distinct chain length acyl-CoAs, catalyzed by carnitine acyltransferases of distinct chain length specificities (carnitine acetyl-, octanoyl-, and palmitoyltranferases) and in the transportation of activated fatty acids through membrane systems within the cell, particularly into the mitochondrial matrix (long-chain fatty acid oxidation, known as mitochondrial β-oxidation) (Kerner and Hoppel, 2013). This latter process represents the repetitive oxidative cleavage of long-chain fatty acids into two carbon units, acetyl-CoA, which is further oxidized for energy production. In addition to l-carnitine, the biologically active form, a variety of specific carnitine forms have been studied. Acetyl, propionyl, tartrate, and fumarate are some of the carnitine salts investigated with specific goals. Acetyl-l-carnitine has the ability to cross the blood–brain barrier and has been used for enhancing cognitive function, memory, and mood (Inano et al., 2003). Ho et al. (2010) reported that l-carnitine l-tartrate has an important impact in selected markers of exercise recovery. Synthetized by the esterification of propionic acid and carnitine, the propionyl-l-carnitine (PLC) is a novel form of carnitine with multiple physiological roles which has recently been used as a food supplement in the form of glycine propionyl-l-carnitine (GPLC) (Mingorance et al., 2011).
CARNITINE
The daily requirement of carnitine by humans is met by endogenous synthesis and dietary intake, mostly from meat and meat products. In the former case, protein bound lysine is first methylated to trimethylysine (TML) using s-adenosylmethionine. Availability of the intermediate TML limits carnitine biosynthesis with most TML stored in the body
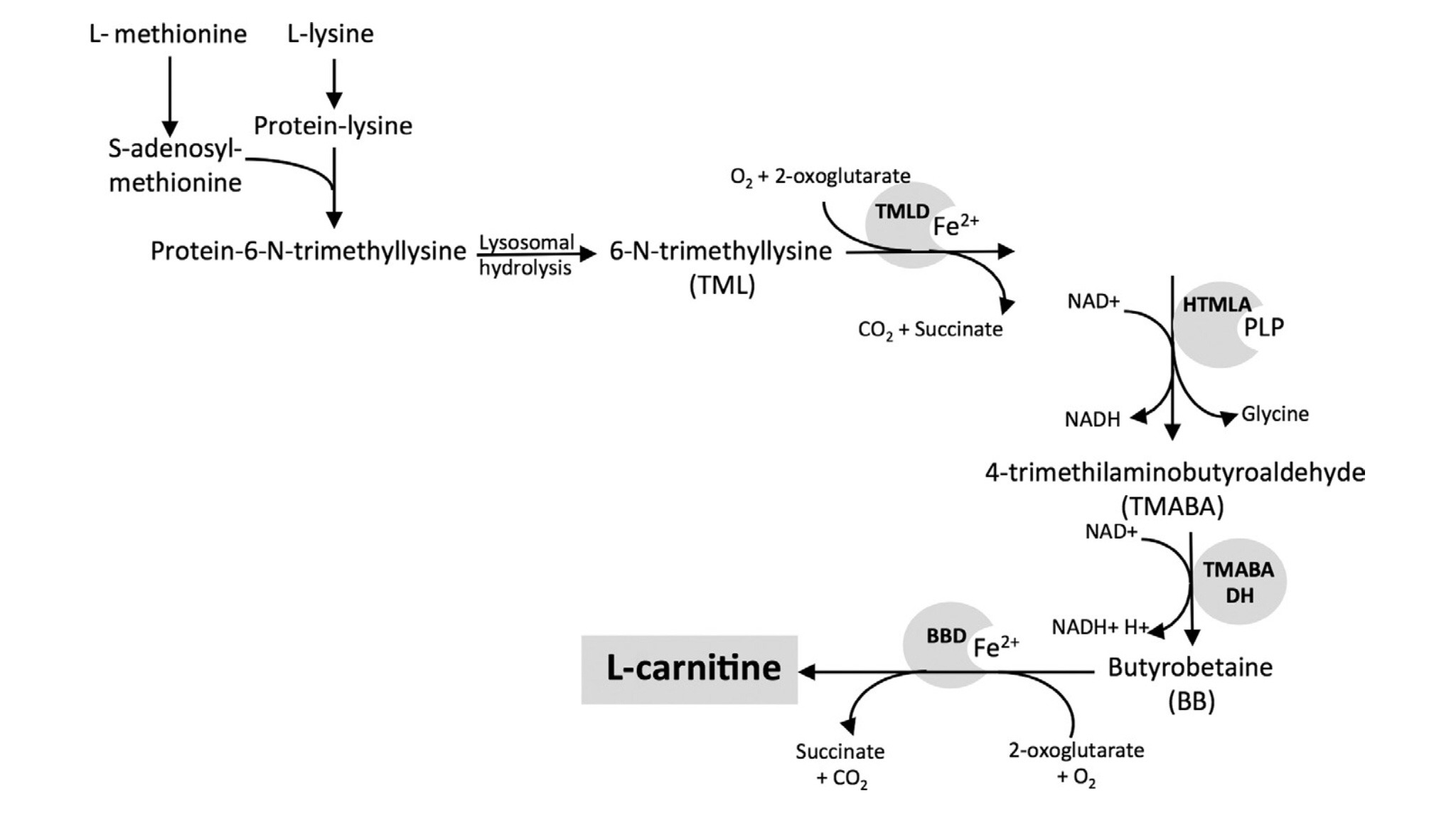
Biosynthesis of L-carnitine
being located in skeletal muscle protein. Following proteolytic liberation, free TML is converted by multiple reactions to butyrobetaine (BB) (not in cardiac and skeletal muscle), the ultimate carnitine precursor. In such process, lysine supplies the carbon skeleton to the carnitine molecule (Fig. 2.5.1) and, in turn, methyl groups come from methionine residue (Pekala et al., 2011). The N-metylation of lysine residues observed in many proteins (myosin and actin for example) is a kind of translational modification with such reaction being catalysed by a specific methyltransferase. s-adenosyl-l-methionine is a cosubstract with chemically reactive methyl groups attached to the sulfur atom, which makes it a methyl-group donor.
The lysossomal hydrolysis of proteins containing TML releases the TML residues, which are then hydroxylated by mitochondrial dioxygenase TML (TMLD) to 3-hydroxytrimethyllysine (HTML). The next stages involve HTML cleavage to glycine and 4-trimethylaminobutyraldehyde (TMABA) catalysed by HTML aldolase (HTMLA) and the dehydrogenation of TMABA to give BB catalysed by TMABA dehydrogenase (TMABA DH). Although most tissues are capable of synthesizing BB, the hydroxylation of BB to carnitine is restricted to the liver and, to a lesser extent, in the kidneys and the brain (Berardi et al., 1998; Kerner and Hoppel, 2013), requiring the iron ion and ascorbate as cofactors (Paik et al., 1977; van Vlies et al., 2006).

Free l-carnitine, absorbed from dietary intake or synthesized in the liver and kidneys, reaches the blood stream and is then taken up by other tissues. Since the carnitine concentration in tissues is generally higher than in plasma, its body distribution is determined by a series of systems of active transport against a concentration gradient, an independent efflux process, and an exchange mechanism, specific to each tissue type. Under physiological conditions, plasma carnitine concentration is maintained within a narrow range by a modest rate of inner carnitine synthesis, dietary intake, and efficient management by the kidneys (Kerner and Hoppel, 2013). Carnitine is not metabolized in the human body, being filtered at the renal glomerulus with about 85% of it being reabsorbed by the proximal tubules (Rebouche and Engels, 1984).
Less than 2% of the absorbed carnitine is excreted in urine or bile (Pekala et al., 2011), in the form of l-carnitine, acetyl l-carnitine, and other acylcarnitine esters (Rebouche and Engels, 1984). Nevertheless, longer chain carnitine esters are absorbed less than the other carnitine forms. Because tissues such as heart, muscle, liver, and kidney are very dependent on the energy generated by β-oxidation, it is crucial they have sufficient amounts of carnitine.
DIETARY SOURCES AND INTAKE OF l-CARNITINE
The carnitine reserves consist of nonesterified molecules (free carnitine) and multiple acylcarnitine esters (forms bounded
to different fatty acids). About 99.5% of body carnitine is intracellular, while circulating plasma carnitine accounts for only 0.5%. Daily urinary carnitine excretion equals the sum of dietary absorption and endogenous synthesis (about 400 μmol/ day) (El-Hattab and Scaglia, 2015; Rebouche, 1992; Stanley, 2004).
The body carnitine level is maintained by absorption from the diet, synthesis, and renal reabsorption. At normal physiological
conditions, renal carnitine reabsorption is very efficient (90%–99% of the filtered load) being equal to the normal plasma carnitine concentration (approximately 50 μmol/L). Thus, when carnitine increases in the plasma circulation, the efficiency of its reabsorption decreases and its clearance increases, which results in a rapid decline of carnitine concentration to its baseline. Therefore, as the dietary intake of carnitine varies, urinary carnitine excretion also varies to keep plasma carnitine within the normal range (Ramsay et al., 2001). Under normal circumstances, an adult (about 70 kg) can synthetize from 11 to 34 mg of l-carnitine each day (160–480 μg/kg body weight). This amount can be insufficient when living is stressful or physically exigent, namely in the case of men undertaking advanced sports training or athletes. About
75% of the carnitine present in the body is obtained from the diet (Flanagan et al., 2010). l-carnitine is mostly present in meats and dairy products and almost absent in vegetables (Rebouche et al., 1993). Among foods from animal origins, lamb and beef have higher l-carnitine contents than fish, pork, and poultry, followed by, in decreasing order, whole milk and cottage cheese. In fruits and vegetables, only avocado and asparagus have noteworthy amounts of carnitine (Pekala et al., 2011). Since carnitine is more concentrated in animal products, strict vegetarians, and lacto-ovo vegetarians, get very little carnitine from their diets. The rate of l-carnitine biosynthesis in vegetarians is estimated to be around 1.2 μmol/kg of body weight per day while omnivorous humans ingest 2–12 μmol/kg of body weight per day, which represents 75% of carnitine sources in the body (Vaz and Wanders, 2002). Regular supplementation is sometimes recommended but, in theory, makes sense only in individuals performing acute physically stressful tasks (muscle carnitine faster depletion). The bioavailability
of oral carnitine dietary supplements is only in the order of 14%–18% of the dose (Rebouche, 2004).
CARNITINE FUNCTIONS
There are two forms of carnitine: l-carnitine (biologically active) and d-carnitine (inactive). Aside from the assistance in fatty acid transport, l-carnitine and its derivative salts (fumarate, acetyl, tartrate, propionyl, etc.) show antioxidant activity (Calo et al., 2006) and may participate in improving cognitive function (acetyl-l-carnitine) (Inano et al., 2003), exercise recovery (l-carnitine l-tartrate), (Ho et al., 2010) and nitric oxide (NO) production (PLC; GPLC) (Mingorance et al., 2011).
The main function of carnitine in intermediary metabolism is the transport of long-chain fatty acids from the cytosol to the mitochondrial matrix. l-carnitine is yet involved in the transfer of peroxisomal β-oxidation products (acetyl-CoA) for Krebs cycle oxidation or in the modulation of the acyl-CoA/CoA ratio, storage of energy as acetyl-carnitine, and regulation of the toxic effects of poorly metabolized acyl groups by excreting them as carnitine esters (excretion in urine) (Pekala
et al., 2011).

CELLULAR UPTAKE AND ACTIVATION OF LONG-CHAIN FATTY ACIDS
Long-chain fatty acids represent an unequivocal source of energy production for many organs, mainly for muscle and liver, but since most tissues have only residual levels of storage lipids, they depend on a continuous supply of fatty acids from adipose tissue following mobilization by lipolysis and transport in the blood bound to albumin. The fatty acids uptake by tissues is a process mediated by transport proteins located in the plasmatic membrane and once within the cell they are then bound to proteins existing in considerable amounts in the cytosol. Depending on the tissue demand for energy, fatty acids are transformed to triglycerides and stored for further oxidation in mitochondria. Before being sent into storage or oxidation, fatty acids are first activated to acyl-CoA esters, with such reactions being catalyzed by long-chain acyl-CoA synthetase.
Cytosolic long-chain acyl-CoA is impermeable to the mitochondrial membranes and, in general, carnitine works as a carrier for the acyl groups. Long-chain fatty acid acyl groups are transported exclusively as carnitine esters by translocase, which constitutes a transmembraneous protein located in the inner mitochondrial membrane. The impermeability of the mitochondrial membranes, particularly the outer membrane, can be overcome by a voltage-dependent mechanism, involving an anion-selective channel, called mitochondrial porin, which regulates the permeability of this membrane to ions and metabolites (Kerner and Hoppel, 2013).
The mitochondrial carnitine system plays a crucial role in the β-oxidation of long-chain fatty acids through their transport into the matrix, involving the malonyl-CoA-sensitive carnitine palmitoyltransferase-I (CPT-I, located in the outer membrane), the carnitine:acylcarnitine translocase (CACT) (an integral inner membrane protein), and carnitine
palmitoyltransferase-II (CPT-II, localized on the matrix side of the inner membrane) (Fig. 2.5.3). CPT-I transfers activated long-chain acyl residues from acyl-CoA into carnitine. The resulting long-chain acylcarnitine esters are transported over the inner mitochondrial membrane via an integral inner membrane protein, CACT. Following the
translocation of long-chain acylcarnitines into the mitochondrial matrix, the carnitine esters are converted to their respective intramitochondrial CoA esters by CPT-II, thus completing the carnitine-dependent uptake of activated fatty acids (Longo et al., 2016). Finally, the acyl-CoA undergoes β-oxidation with a release of energy in the ATP form. Fatty acid β-oxidation is a multistep process by which the activated long-chain fatty acids are broken down, with each cycle
resulting in the removal of two carbon atoms from the fatty acyl residue in the form of acetyl-CoA (Kerner and Hoppel, 2013; Pekala et al., 2011).
The influence of l-carnitine on an exercise-altered metabolism may be explained by its relation to acetyl-CoA. Acetyl- CoA is a common product of glycolysis and fatty acid l-carnitine β-oxidation. Increased levels of acetyl-CoA can interfere with the conversion of pyruvate into acetyl-CoA by suppressing the l-carnitine-mediated increase (Kerner and Hoppel, 2013).

Main functions of carnitine in the brain, liver, and muscle cells under physiological conditions

Role of L-carnitine in the transport of long chain fatty acid into the mitochondria. CACT, carnitine-acylcarnitine translocase; CAT, carnitine acetyltransferase; CPT I, carnitine palmitoyltransferase I; CPT II, carnitine palmitoyltransferase II
l-CARNITINE’S ANTIOXIDANT ROLE DURING OXIDATIVE STRESS
Despite the role of l-carnitine in fatty acid transport, many studies have suggested this compound as an antioxidant (Lohninger et al., 2005; Ribas et al., 2014; Surai, 2015). This role of carnitine seems to be an apparent contradiction, since l-carnitine increases the metabolism of fatty acids facilitating the formation of reactive oxygen species (ROS) by the electron transport chains of mitochondria. However, it has been reported that l-carnitine determines the formation of NO (Brown, 1999), activating oxidative damage defense enzymes (Kremser et al., 1995) and superoxide dismutase (SOD) as well as catalase against 3-nitropropionic acid-induced neurotoxicity (Kremser et al., 1995). According to the literature review we can conclude that there are several important mechanisms in the antioxidant action of carnitine (Bloomer et al., 2013; Kolodziejczyk et al., 2011; Ribas et al., 2014; Sung et al., 2016; Surai, 2015). l-carnitine is shown to directly scavenge free radicals and it can chelate transition metals (Fe2+ and Cu+), preventing their participation in ROS formation (Surai, 2015). l-carnitine decreases free radical formation by inhibiting specific enzymes (e.g., xanthine oxidase and
NADPH oxidase) responsible for free radical production, which have a high biological relevance in various stress conditions.
In addition, carnitine participates in maintaining the integrity of mitochondria, including the electron-transport chain of mitochondria, in stress conditions. Indeed, carnitine can be considered as a mitochondria-specific antioxidant, responsible for the maintenance of mitochondria integrity and regulation of ROS production and ROS signaling (Surai, 2015). The protective effect of l-carnitine and its derivatives on the antioxidant systems of the body is also shown in various models of oxidative stress/toxicity caused by a variety of toxicants and neurotoxic agents (Surai, 2015).

Determined under in vitro conditions, the antioxidant capacity of l-carnitine seems to be dependent on concentration, behaving similarly to α-tocopherol and trolox administered at a concentration of 30 μM, through scavenging effects (Gülçin, 2006). l-carnitine administration during exercise is expected to boost the activity of endogenous antioxidants, delaying fatigue by removing ROS (Wickens, 2001). In a study using human blood samples it was concluded that l-carnitine provided protective effects, including suppressing peroxynitrite-induced peroxidation and decreasing low molecular–weight thiols, glutathione and cysteine, through the oxidation of the arachidonic acid cascade and antioxidant mechanisms (Malaguarnera et al., 2009; Saluk-Juszczak et al., 2010).
Low-density lipoprotein (LDL) cholesterol is one of the major risks for cardiovascular diseases (Lembo et al., 2000), with the oxidized form being an essential element in atherosclerotic plaque formation (Boullier et al., 2001; Steinberg, 1997). Oral administration of l-carnitine in patients with diabetes with increased oxidized LDL levels reduced oxidized LDL, indicating that l-carnitine can effectively control diseases induced by ROS increase. Thus, l-carnitine is effective in a relatively wide range of ROS and ROS-induced lipid peroxidation, preventing inflammation by scavenging mechanisms. However, the precise
mechanism by which l-carnitine acts as an antioxidant has not yet been confirmed (Boullier et al., 2001).
The increase of ATP synthesis by the electron transport chain and the production of ROS associated with physical exercise, promotes reduced muscle contraction, inducing fatigue and loss of performance. The administration of l-carnitine improves exercise performance since it accelerates ATP synthesis by fatty acid metabolism, removes and mutates ROS, and activates stabilization of endogenous antioxidants, improving muscle contraction efficiency and delaying fatigue (Sung
et al., 2016).
OTHER ROLES OF CARNITINE IN METABOLISM
l-carnitine plays a crucial role in the maintenance of the acetyl CoA/CoA ratio in the cell during high-intensity exercise, which produces large amounts of acetyl CoA (Hoppel, 2003; Pekala et al., 2011). Such an increase inhibits the pyruvate dehydrogenase complex and consequently the rise of lactate. By reacting with acetyl-CoA carnitine suppresses the accumulation of lactic acid, forming acetyl carnitine and CoA, enhancing performance under high-intensity exercise. In some other metabolic conditions, for example, ischemia, fasting, and acute stress, characterized by increased pyruvate dehydrogenase activity and fatty acid supply from activated lipolysis, the capacity to oxidize acetyl-CoA may be exceeded, leading to an accumulation of acetyl-CoA and short chain acyl-CoA esters obtained from the degradation of branched-chain amino acids in skeletal muscle (Pekala et al., 2011).
Carnitine is also an activator of carbohydrate metabolism by promoting pyruvate oxidation associated with the decrease in acetyl-CoA content (Pekala et al., 2011).
SUPPLEMENTATION OF l-CARNITINE IN SPORTS NUTRITION
The performance enhancement of l-carnitine on exercise is due to glycogen-sparing effects, reduction in the accumulation of lactate, and an increase in fatty acid metabolism. However, the increased accumulation of ROS deteriorates the force of muscle contraction as well as the oxidation level of plasma components. Ergonomic aids in sports nutrition include dietary antioxidants such as vitamin C and E to improve exercise performance, by reducing oxidative stress (Bryant et al., 2003; Snider et al., 1992; Sung et al., 2016). Based on the few studies carried out so far, it is difficult to determine the optimal dosage of l-carnitine as well as its administration period. l-carnitine is probably beneficial when muscular function is impaired in metabolic diseases, but has little or no effect in healthy individuals. While exercise increases the metabolic rate, it can result concomitantly in excessive ROS formation. In such cases l-carnitine aids by reducing oxidative stress by attenuating ROS and accelerating endogenous antioxidant activity.

POTENTIAL EFFECT OF CARNITINE AS A THERAPEUTIC AGENT
It is well known that l-carnitine and its esters are able to improve metabolic functions, inclusively under pathological conditions (Nagesh et al., 2011; Ramsay and Zammit 2004; Shenk et al., 2009; Zhang et al., 2010).
The supplementation of l-carnitine seems to benefit conditions such as anorexia, chronic fatigue, coronary vascular disease, hypoglycemia, male infertility, and muscular myopathies, among others (Pekala et al., 2011). Clinical studies have demonstrated that l-carnitine favorably modulates oxidative stress through preventing membrane fatty acid peroxidation
(Malaguarnera et al., 2009). According to Sayed-Ahmed et al. (2001) l-carnitine prevented the progression of atherosclerotic lesions. The protective effects of l-carnitine against damage to the heart, caused by diabetes-induced alterations, and additional ischemia have been described by Schneider et al. (2005). l-carnitine may be an important agent in the protection of myocardial alterations in diabetes with additional ischemia, since it stabilizes mitochondrial and cellular functions and acts through its antioxidant or radical scavenging potential (Kolodziejczyk et al., 2011).
As a food supplement, carnitine is mostly available as l-carnitine or bound to either acetic or propionic acids (acetyl l-carnitine and propionyl l-carnitine, respectively). Acetyl-l-carnitine (ALC) is produced from l-carnitine and acetyl-CoA in mitochondria by carnitine O-acetyltransferase, and transported to the cytoplasm where it is converted back to l-carnitine and acetyl-CoA. Several studies have suggested that ALC may play a neuroprotective role in hypoxic-ischemic brain injury (Virmani and Binienda, 2004; Wainwright et al., 2006; Zanelli et al., 2005). ALC serves as a source of acetylcholine and l-glutamate, and also contributes to energy-producing reactions. The ALC appears to be the best form to use for brain disorders (Alzheimer’s disease) while propionyl l-carnitine seems to be more effective for heart and peripherical vascular diseases. PLC is a naturally occurring derivative of carnitine that plays an important role in the metabolism of both carbohydrates and lipids, leading to an increase of ATP generation. PLC is transported, into the cell, to the mitochondria, where it is transformed into free carnitine and propionyl-CoA. The latter is converted into succinyl-CoA and finally to succinate, which is involved in the citric acid cycle. PLC is also a potent antiradical agent and thus may protect tissues from oxidative damage. PLC has been demonstrated to exert a protective effect in different models of both cardiac and endothelial dysfunction, to prevent the progression of atherosclerosis, and, more recently, to improve some of the cardiometabolic alterations
that frequently accompany insulin resistance (Mingorance et al., 2011). PLC is a novel carnitine molecule known in the dietary supplements sector as GPLC. Both PLC and GPLC have been reported to improve the physical condition, with increased nitric oxide metabolites (Bloomer et al., 2007, 2009).

CONCLUSIONS
l-carnitine is an amino acid derivative, available in several forms, which possesses multiple physiological properties. The main known functions of l-carnitine are the transport of activated long-chain fatty acids from the cytosol to the mitochondrial matrix, the modulation of the acyl CoA/CoA ratio, storage of energy as acetyl-carnitine, the modulation of the toxic effects of poorly metabolized acyl groups, and its antioxidant activity. Several studies have demonstrated the antioxidant properties for l-carnitine in different pathologies such as diabetes, hypertension, renal and liver diseases, and also in neurodegenerative conditions. l-carnitine, as a nutritional supplement, has been considered a promising candidate for the prevention and treatment of oxidative alterations in many metabolic diseases. Concerning the optimal dosage and route
of administration, additional, well-controlled studies are still needed to clarify safe, practical, and therapeutic guidelines.
Acetyl l-carnitine and propionyl l-carnitine, the main esterified forms of l-carnitine, have been studied in terms of its role in enhancing cognitive function, exercise recovery, and in the heart and peripheral vascular system. l-carnitine and/or its esterified forms seem to play an important role in the metabolism of the human body when it can be used as a therapeutic agent. l-carnitine supplementation may be useful not only to prevent tissue deficiency, but also to avoid oxidative damage,
secondary to an increased production of ROS. Considering the ability of l-carnitine to easily cross the blood–brain barrier, l-carnitine supplementation may also be beneficial in preventing neurological damage derived from oxidative injury.
However, further studies are required to better explore this potential role.
REFERENCES
Berardi, S., Stieger, B., Wachter, S., O’Neill, B., Krahenbuhl, S., 1998. Characterization of a sodium-dependent transport system for butyrobetaine into
rat liver plasma membrane vesicles. Hepatol. 28, 521–525.
Bloomer, R.J., Smith, W.A., Fisher-Wellman, K.H., 2007. Glycine propionyl-l-carnitine increases plasma nitrate/nitrite in resistance trained men. J. Int.
Soc. Sports Nutr. 4, 22.
Bloomer, R.J., Tschume, L.C., Smith, W.A., 2009. Glycine propionyl-l-carnitine modulates lipid peroxidation and nitric oxide in human subjects. Int. J.
Vitam. Nutr. Res. 79, 131–141.
Bloomer, R.J., Farney, T.M., McAllister, M.J., 2013. An overview of carnitine. Nutrition and Enhanced Sports Performance. Elsevier Academic Press,
New York. pp. 405–413.
Boullier, A., Bird, D.A., Chang, M.K., Dennis, E.A., Friedman, P., Gillotre-Taylor, K., Hörkkö, S., Palinski, W., Quehenberger, O., Shaw, P., Steinberg, D.,
Terpstra, V., Witztum, J.L., 2001. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann. N. Y. Acad. Sci. 947, 214–922.
Bremer, J., 1983. Carnitine-metabolism and functions. Physiol. Rev. 63, 1420–1480.
Brown, G.C., 1999. Nitric oxide and mitochondrial respiration. Biochim. Biophys. Acta 1411, 351–369.
Bryant, R.J., Ryder, J., Martino, R., Kim, J., Craig, B.W., 2003. Effects of vitamin E and C supplementation either alone or in combination on exerciseinduced
lipid peroxidation in trained cyclists. J. Strength Cond. Res. 17, 792–800.
Calo, L.A., Pagnin, E., Davis, P.A., Semplicini, A., Nicolai, R., Calvani, M., Pessina, A.C., 2006. Antioxidant effect of l-carnitine and its short chain
esters: relevance for the protection from oxidative stress related cardiovascular damage. Int. J. Cardiol. 107, 54–60.
Carter, A.L., Abney, T.O., Lapp, D.F., 1995. Biosynthesis and metabolism of carnitine. J. Child Neurol. 10 (Suppl. 2), S3–S7.
Duran, M., Loof, N.E., Ketting, D., Dorland, L., 1990. Secondary carnitine deficiency. J. Clin. Chem. Clin. Biochem. 28, 359–363.
El-Hattab, A.W., Scaglia, F., 2015. Disorders of carnitine biosynthesis and transport. Mol. Genet. Metab. 116 (3), 107–112.
Flanagan, J.L., Simmons, P.A., Vehige, J., Willcox, M.D.P., Garrett, Q., 2010. Role of carnitine in disease. Nutr. Metab. 7, 30–44.
GülçinF I., 2006. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006 (78), 803–811.
Ho, J.Y., Kraemer, W.J., Volek, J.S., Fragala, M.S., Thomas, G.A., Dunn-Lewis, C., 2010. Carnitine l-tartrate supplementation favorably affects biochemical
markers of recovery from physical exertion in middle-aged men and women. Metabolism 59 (8), 1190–1199.
Hoppel, C., 2003. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 41 (4 Suppl 4), S4–S12.
Inano, A., Sai, Y., Nikaido, H., Hasimoto, N., Asano, M., Tsuji, A., Tamai, I., 2003. Acetyl-l-carnitine permeability across the blood–brain barrier and
involvement of carnitine transporter OCTN2. Biopharm. Drug Dispos. 24, 357–365.
Kerner, J., Hoppel, C.L., 2013. Carnitine and β-oxidation. Metabolism Vitamins and Hormones. Elsevier. pp. 384–387.
Kolodziejczyk, J., Saluk-Juszczak, J., Wachowicz, B., 2011. L-carnitine protects plasma components against oxidative alterations. Nutrition 27, 693–699.
Kremser, K., Stangl, H., Pahan, K., Singh, I., 1995. Nitric oxide regulates peroxisomal enzyme activities. Eur. J. Clin. Chem. Clin. Biochem. 33, 763–774.
Lembo, G., Vecchione, C., Izzo, R., Fratta, L., Fontana, D., Marino, G., Pilato, G., Trimarco, B., 2000. Noradrenergic vascular hyper-responsiveness in
human hypertension is dependent on oxygen free radical impairment of nitric oxide activity. Circulation 102, 552–557.
Lohninger, A., Pittner, G., Pittner, F., 2005. l-carnitine: new aspects of a known compound—a brief survey. Montsh. Chem. 136, 1255–1268.
Longo, N., Frigeni, M., Pasquali, M., 2016. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 1863, 2422–2435.
Malaguarnera, M., Vacante, M., Avitabile, T., Malaguarnera, M., Cammalleri, L., Motta, M., 2009. l-carnitine supplementation reduces oxidized LDL
cholesterol in patients with diabetes. Am. J. Clin. Nutr. 89, 71–76.
McGarry, J.D., Brown, N.F., 1997. The mitochondrial carnitine palmitoyltransferase system from concept to molecular analysis. Eur. J. Biochem.
244, 1–14.
Mingorance, C., Rodríguez-Rodríguez, R., Justo, M.L., María Álvarez de Sotomayor, M.A., Herrera, M.D., 2011. Critical update for the clinical use of
l-carnitine analogs in cardiometabolic disorders. Vasc. Health Risk Manag. 27, 169–176.
Nagesh, B.G., Kumar, A., Singh, R.L., 2011. Chronic pretreatment with acetyl-l-carnitine and ±DL-α-lipoic acid protects against acute glutamateinduced
neurotoxicity in rat brain by altering mitochondrial function. Neurotox. Res. 19, 319–329.
Paik, W.K., Nochumson, S., Kim, S., 1977. Carnitine biosynthesis via protein methylation. Sci. Trends Biochem. Sci. 2, 159–161.
Pekala, J., Patkowska-Sokoła, B., Bodkowski, R., Jamroz, D., Nowakowski, P., Lochyński, S., Librowski, T., 2011. l-carnitine—metabolic functions and
meaning in humans life. Curr. Drug Metab. 12 (7), 667–678.
Ramsay, R.R., Zammit, V.A., 2004. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol. Aspects Med. 25, 475–493.
Ramsay, R.R., Gandour, R.D., van der Leij, F.R., 2001. Molecular enzymology of carnitine transfer and transport. Biochim. Biophys. Acta. 1546, 21–43.
Rebouche, C.J., 1992. Carnitine function and requirements during the life cycle. FASEB J. 6, 3379–3386.
Rebouche, C.J., 1996. Role of carnitine biosynthesis and renal conservation of carnitine in genetic and acquired disorders of carnitine metabolism. In:
Seim, H., Loster, H. (Eds.), Carnitine: Pathobiochemical Basics and Clinical Applications. Ponte Press, Bochum. pp. 111–121.
Rebouche, C.J., 2004. Kinetics, pharmacokinetics and regulation of l-carnitine and acetyl-l-carnitine metabolism. Ann. N. Y. Acad. Sci. 1033, 30–41.
Rebouche, C.J., Engels, A.G., 1984. Kinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes. J. Clion.
Invest. 73, . 957-867.
Rebouche, C.J., Lombard, K.A., Chenard, C.A., 1993. Renal adaptation to dietary carnitine in humans. Am. J. Clin. Nutr. 58, 660–665.
Ribas, G.S., Vargas, C.R., Wajner, M., 2014. l-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene
533, 469–476.
Saluk-Juszczak, J., Olas, B., Wachowicz, B., Glowacki, R., Bald, E., 2010. l-carnitine modulates blood platelet oxidative stress. Cell Biol. Toxicol. 26,
355–365.
Sayed-Ahmed, M., Khattab, M.M., Gad, M.Z., Mostafa, N., 2001. l-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic
rabbits. Pharmacol. Res. 3, 235–242.
Schneider, R., Löster, H., Aust, W., Craatz, S., Welt, K., Fitzl, G., 2005. Protective effects of l-carnitine on myocardium of experimentally diabetic rats
with additional ischaemia and reperfusion. Monatsh Chem. 136, 1467–1481.
Shenk, J.C., Liu, J., Fischbach, K., Xu, K., Puchowicz, M., Obrenovich, M.E., Gasimov, E., Alvarez, L.M., Ames, B.N., Lamanna, J.C., Aliev, G., 2009. The
effect of acetyl-l-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer’s disease. J. Neurol. Sci. 283, 199–206.
Snider, I.P., Bazzarre, T.L., Murdoch, S.D., Goldfarb, A., 1992. Effects of coenzyme athletic performance system as an ergogenic aid on endurance performance
to exhaustion. Int. J. Sport Nutr. 2, 272–286.
Stanley, C.A., 2004. Carnitine deficiency disorders in children. Ann. N. Y. Acad. Sci. 1033, 42–51.
Steinberg, D., 1997. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 272, 20963–20966.
Sung, D.J., Kim, S., Kim, J., An, H.S., So, W.-Y., 2016. Role of l-carnitine in sports performance: focus on ergogenic aid and antioxidant. Sci. Sports.
31, 177–188.
Surai, P.F., 2015. Carnitine enigma: from antioxidant action to vitagene regulation. Part 1. Absorption, metabolism, and antioxidant activities. J. Veter.
Sci. Med. 3 (2), 14.
van Vlies, N., Wanders, R.J., Vaz, F.M., 2006. Measurement of carnitine biosynthesis enzyme activities by tandem mass spectrometry: differences between
the mouse and the rat. Anal. Biochem. 354, 132–139.
Vaz, F.M., Wanders, R.J.A., 2002. Carnitine biosynthesis in mammals. Biochem. J. 361, 417–429.
Virmani, A., Binienda, Z., 2004. Role of carnitine esters in brain neuropathology. Mol. Aspects Med. 25, 533–549.
Wainwright, M.S., Kohli, R., Whitington, P.F., Chace, D.H., 2006. Carnitine treatment inhibits increases in cerebral carnitine esters and glutamate detected
by mass spectrometry after hypoxia-ischemia in newborn rats. Stroke 37, 524–530.
Wickens, A.P., 2001. Aging and the free radical theory. Respir. Physiol. 128, 379–391.
Zanelli, S.A., Solenski, N.J., Rosenthal, R.E., Fiskum, G., 2005. Mechanisms of ischemic neuroprotection by acetyl-l-carnitine. Ann. N. Y. Acad. Sci.
1053, 153–161.
Zhang, H., Jia, H., Liu, J., Ao, N., Yan, B., Shen, W., Wang, X., Li, X., Luo, C., Liu, J., 2010. Combined R-α–lipoic acid and acetyl-l-carnitine exerts
efficient preventative effects in a cellular model of Parkinson’s disease. J. Cell Mol. Med. 14, 215–225.
